Clinical trials are the engine of medical advancement, pushing the boundaries of knowledge and bringing innovative treatments to light. As we look toward 2025, specific trials stand out as possible game-changers.
These studies don’t just promise new drugs or procedures; they represent transformative approaches to treating disease, improving quality of life, and expanding access to care.
Here, we’ll highlight three clinical trials poised to significantly impact healthcare in 2025, shaping the medical landscape and redefining what’s possible in patient outcomes.
1. Groundbreaking Gene Therapy for Rare Diseases
One of the most exciting clinical trials underway involves gene therapies that could address rare, previously untreatable conditions. Building on recent FDA approvals, researchers are now exploring gene-editing technologies like CRISPR to repair genetic mutations at their source. This approach could lead to long-term improvements—or potentially even lasting solutions—for conditions such as Duchenne muscular dystrophy or certain forms of inherited blindness. If successful, these trials will set a precedent for treating rare diseases, shifting the focus from managing symptoms to achieving proper, lasting recovery.
Beyond the direct benefit to patients, these gene therapy trials carry implications for the broader healthcare industry. By demonstrating the potential of gene-editing methods, these trials could encourage further research into other genetic conditions and contribute to discussions on regulatory pathways. As more practitioners and researchers gain confidence in these methods, the development of similar therapies will accelerate, transforming the treatment landscape for years.

Gene therapy clinical trials carry significant implications for the broader healthcare industry.
2. Immunotherapy Advances for Cancer Treatment
Immunotherapy continues to be one of the most transformative areas in oncology, and ongoing trials in 2025 are exploring how to broaden its applicability and improve response rates. While checkpoint inhibitors and CAR-T cell therapies have already changed how we think about cancer treatment, researchers are now looking into combination therapies—pairing immunotherapy agents with targeted drugs or radiation.
Early results suggest these combinations may improve response rates in certain cancers that are traditionally resistant to standard therapies, including aggressive forms of pancreatic, ovarian, and lung cancers.
What makes these trials particularly noteworthy is their potential to refine treatment protocols and reduce side effects. By better understanding how immune cells can be directed and controlled, these studies could lead to more personalized approaches to cancer care.
These advances could lead to more efficient therapies with faster response times for the healthcare system and professionals in clinical research training.
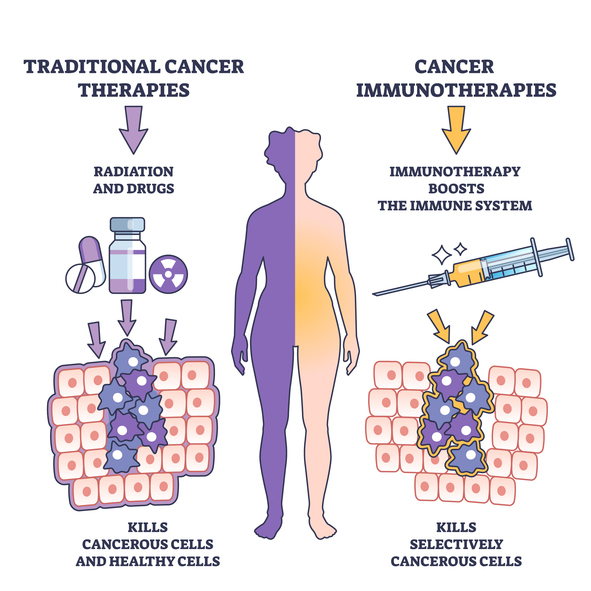
Immunotherapy clinical trials have the potential to refine treatment protocols and reduce side effects.
3. Expanding Access Through Decentralized Clinical Trials
In addition to groundbreaking medical interventions, clinical trials are also evolving in how they are conducted. Decentralized or virtual clinical trials are a trend that could redefine participation and expand access. By leveraging digital technologies – such as wearable health trackers, remote patient monitoring, and telemedicine – these trials allow participants to contribute data without travelling to a central location.
In 2025, several high-profile trials are expanding the use of this model, particularly in rural areas or regions with limited healthcare access, though regulatory and technological challenges remain.

Clinical trials are evolving based on how they are being conducted.
This shift could have profound implications for global healthcare. By reducing logistical barriers, decentralized trials can recruit more diverse populations, leading to data that better reflects the real-world efficacy of treatments.
For participants, it means greater convenience, fewer disruptions to their daily lives, and potentially faster enrollment. It translates to higher retention rates and more robust data sets for researchers, accelerating the timeline from study to approval. As decentralized trials gain momentum, they could significantly improve equity and efficiency in clinical research, though complete standardization is still in progress.
Are you interested in obtaining a clinical research diploma?
Contact AAPS for more information.




